All life shares the same carbon metabolism. However, enzymes differ, reflecting ancient evolutionary roots. Interestingly, the enzymes that produce glucose and glycogen are more conserved in both archaea and bacteria than the enzymes that break down glucose. That’s why the review “The early evolution of the glycolytic pathway from autotrophic origins to glycogen and back” in FEMS Microbiology Reviews argues that the metabolic pathway of glycolysis arose from gluconeogenesis in autotrophs. In this #FEMSmicroBlog, we interviewed Luca David Modjewski, one of the co-authors of the article, to walk us through this research topic. #FascinatingMicrobes
Why is this paper significant for microbiologists outside your field?
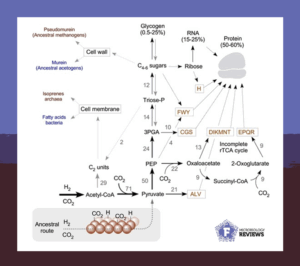
Sugars are the autobahn of carbon metabolism. They shuttle carbon from the environment into the cell interior; through the TCA cycle and energy metabolism, through amino acid and nucleic acid syntheses, and finally into storage.
Our review shows that it has always been that way, even though metabolism started in an autotrophic manner: from carbon dioxide and hydrogen. The enzymes that turn inorganic hydrogen and carbon dioxide into organic three-carbon sugars are universally present in archaea and bacteria.
Further enzymatic conversions generate six-carbon sugars, like glucose. The first free-living cells used these compounds to both synthesize the cell wall and nucleic acids, and store carbon in the form of glycogen.
Hence, it seems that the last universal common ancestor, LUCA, produced simple carbon backbones essential for amino acid and cofactor syntheses and energy storage. This traces gluconeogenesis to the onset of metabolic evolution.
What about the evolution of the carbon metabolism do you find fascinating?
I have been working on various aspects of early biochemical evolution for my PhD thesis. Everything comes back to autotrophic origins, the acetyl-CoA pathway, acetogens and methanogens.
Methanogens synthesize and utilize glycogen, which functions as the reserve polysaccharide made of glucose. Yet, methanogens cannot grow on glucose in culture – how interesting is that!
We propose a potential solution to this puzzle. The first methanogens were unable to compete with fast, versatile fermenters and other organisms that efficiently used environmental substrates like glucose. In contrast, methanogens generate energy by synthesizing methane from hydrogen and carbon dioxide using a methyl transport chain.
This means methanogens remain best adapted to life on hydrogen and carbon dioxide, where no other microbes can outcompete them. Instead of mobilizing environmental glucose, they produce it through gluconeogenesis. They then use their internal glycogen stores through glycolysis to get energy at their own pace.
That appears to be their strategy: be competitive and keep internal storage reserves full.
What was one of the main hurdles you encountered, and how did you solve it?
The vast amounts of sequence data that exist for enzymes of sugar phosphate metabolism and their complex patterns of shared sequence similarity are almost impossible to sort through. Fortunately, Prof. Schönheit knows all these enzymes and sequences on a “first name basis”, which really helped me get the information sorted.
Why is the evolution of the carbon metabolism important for society and policymakers?
The findings show that microbes have always planned ahead by saving carbon and energy in the form of chemical compounds for hard times. Even ancient methanogenic archaea were able to organize internal storage, saving carbon as glycogen.
These comparatively simple organisms remind us that we must use our resources efficiently and always make sure that we have plenty of reserves in storage. Therefore, the global carbon budget and energy supply problems that we face now are as old as the first single-celled organisms and life itself. Life’s solution is simple: use resources wisely.
- Read the review “The early evolution of the glycolytic pathway from autotrophic origins to glycogen and back” by Modjewski et al. in FEMS Microbiology Reviews (2025).

Luca David Modjewski completed a Bachelor’s and Master’s degree at the Institute of Molecular Evolution. Currently in his fifth year of his PhD with Prof. Dr. William F. Martin, he focuses on the bioinformatic examination of prokaryotic metabolic pathways, specifically the oxygen-independent synthesis of vitamin B12, glycolysis and gluconeogenesis, in the context of early evolution starting from hydrogen and carbon dioxide.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |
