DNA damage is a challenge faced by organisms of all domains. To maintain genome integrity, organisms thriving in extreme habitats, such as many archaea, rely heavily on repair pathways. The study “CRISPR–Cas induced self-targeting identifies key players in archaeal microhomology-mediated end joining” in microLife aimed to shed light on how the halophile model organism Haloferax volcanii repairs DNA double-strand breaks fast and efficiently, as explained in this #FEMSmicroBlog by Anna-Lena Sailer. #FascinatingMicrobes
How Haloferax volcanii repairs double-strand breaks
In their extreme habitats, archaea are exposed to many stresses that can cause double-strand breaks. These are among the most serious types of DNA damage that can occur in any organism.
Although archaea have developed robust repair mechanisms, the molecular details of these repair mechanisms remain largely unexplored. For example, it is suspected that the model organism Haloferax volcanii repairs double-strand breaks with two mechanisms: microhomology-mediated end joining and homologous recombination.
The fast and energy-efficient mechanism of microhomology-mediated end joining begins with the exposed regions surrounding the double-strand break. These are homologous to one another and thus anneal. Afterwards, the remaining flaps are removed, the gap is closed by a DNA polymerase and finally, ligation restores genome integrity. However, the proteins likely involved in microhomology-mediated end joining are currently only predicted based on homology to proteins from other organisms and their DNA repair pathways.
Using self-targeting to investigate DNA repair in Haloferax volcanii
The study “CRISPR–Cas induced self-targeting identifies key players in archaeal microhomology-mediated end joining” in microLife aimed to investigate which proteins are associated with this DNA repair mechanism in Haloferax volcanii.
For this, they took advantage of Haloferax volcanii tolerating self-targeting by its own CRISPR-Cas system. They induced DNA damage by CRISPR-Cas at a defined site in the gene crtI encoding a non-essential protein. DNA damage was induced in strains carrying deletions whose gene products are potentially involved in the DNA repair process.
If a cell containing a gene deletion survived, the tested gene product was likely non-essential for the DNA repair process. In the other case, the DNA damage would remain, and the cell would die.
About the proteins involved in DNA repair
This study confirmed that eight proteins are involved in microhomology-mediated end joining as a DNA repair mechanism. The absence of some proteins, namely Cas1, Fen1, Hel308a, PolB1 and LigN, had more striking effects due to significantly reduced survival rates.
Some of the identified proteins, such as Hel308a and Cas1, have never been linked to microhomology-mediated end joining before. While Cas1 is a well-characterised part of the adaptation module of the CRISPR-Cas system, Hel308a is a helicase involved in homologous recombination.
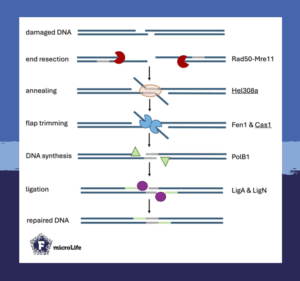
Interestingly, in all tested deletion strains, microhomology-mediated end joining still took place. These results indicate that none of the tested proteins were essential for the repair process.
This suggests that additional back-up proteins may be involved in microhomology-mediated end joining in Haloferax volcanii. The sheer number of proteins assigned to this essential mechanism highlights how seriously archaea take DNA repair.
In conclusion, this study represents an important first step into experimental research on the unique archaeal DNA repair mechanism microhomology-mediated end joining. Evoking DNA lesions at defined sites to pinpoint proteins involved in the damage repair process advances the entire field of archaeal research.
- Read the article “CRISPR–Cas induced self-targeting identifies key players in archaeal microhomology-mediated end joining” by Sailer et al. in microLife (2025).

Anna-Lena Sailer is currently conducting her PhD thesis in the Marchfelder Lab at Ulm University. Anna-Lena´s research focuses on the repair of DNA damages as well as the CRISPR-Cas system in the archaeon Haloferax volcanii. Her interest in CRISPR-Cas based modifications and RNA biology has already arisen during her studies of molecular biotechnology in Heidelberg. However, she has since switched from human disease related projects to basic research in archaea, one of the most unexplored but interesting domains.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |

