#FEMSmicroBlog: Double trouble: How a Mini-Me endolysin isoform supercharges bacterial lysis
Phages – viruses that infect and kill bacteria – carry a specialised toolkit to complete their life cycles and leave their bacterial hosts. Among the most important of these tools are endolysins; enzymes that rapidly break down bacterial cell walls so produced viruses can burst out and spread to new targets. Because of their potency and precision, endolysins are attracting significant interest as antimicrobial enzymes in the fight against antimicrobial resistance and bacterial infections. The study “Heterodimerization of staphylococcal phage φ2638A endolysin isoforms and their functional role in bacterial lysis” in microLife examined the functionality of an endolysin and its isoform to optimize killing of the bacterial hosts. Léa Zinsli and Matthew Dunne explain their work on this #FEMSmicroBlog. #FascinatingMicrobes
About bacteria’s natural enemies
During the later stages of a phage infection, endolysins that target the bacterial peptidoglycan layer are produced. These enzymes cleave specific bonds within the cell wall of the bacterium, eventually weakening and rupturing the cell. Ultimately, this causes the bacterium to lyse and allows newly produced phages to burst out and infect neighbouring bacteria.
Even without the producing phage present, endolysins retain their enzymatic ability to rapidly break down bacterial cell walls. This makes recombinant endolysins highly attractive as standalone antimicrobial agents to target Gram-positive bacteria like Staphylococcus aureus.
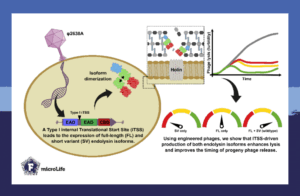
The study “Heterodimerization of staphylococcal phage φ2638A endolysin isoforms and their functional role in bacterial lysis” in microLife investigated the role of endolysins in their natural context during phage infection. For this, we selected the phage φ2638A which infects Staphylococcus pseudintermedius. This particularly intriguing phage produces two isoforms of its endolysin: a full-length version and a shorter “Mini-Me” variant, both derived from the same gene.
The shorter isoform is produced via an internal translational start site in the middle of the endolysin gene. This raised an important evolutionary question: why would this phage produce two similar proteins?
Characterizing staphylococcal endolysin and all its versions
Together with structural biologists, we resolved the three-domain structure of the endolysin. Biochemical experiments found that the two endolysin isoforms interact through their central amidase domains and create a heterodimer composed of one full-length protein and one “Mini-Me”.
To test the importance of producing both isoforms, we genetically engineered phages that expressed only the full-length or the short variant of the endolysin. Both phage versions lysed their bacterial hosts much more slowly than the natural phage, which produced both isoforms.
However, the full-length endolysin alone as a recombinant enzyme, without the presence of the phage, was most potent in killing the bacterial host. Adding the shorter isoform did not enhance its activity.
These results show that the full-length endolysin interacts with its shorter form to improve bacterial lysis during phage infection. The advantage of producing both isoforms is likely specific to the phage infection cycle as it might optimize the efficiency of host cell lysis. This potentially explains why many phages retain internal translational start sites in their endolysin genes to produce Mini-Me isoforms as an evolutionary strategy.
From basic microbiology to future anti-microbial therapies
This project was a true collaborative effort, bringing together microbiologists, structural biologists, protein engineers, and phage experts. It was a lot of fun combining different skills to troubleshoot phage infection assays and collect late-night data at the beamline. But in the end, the collaboration paid off.
By integrating different skills, we uncovered a clever strategy that appears conserved across phages. As viruses contain molecular tools that help improve infection success, we may be able to use them to design smart phages or endolysin-based therapies to tackle bacterial infections and antimicrobial resistance.
Happy World Phage Day!
- Read the article “Heterodimerization of staphylococcal phage φ2638A endolysin isoforms and their functional role in bacterial lysis” by Zinsli et al. in microLife (2025).

Léa Zinsli is a postdoctoral researcher at the University of Montréal, where she investigates phage–host interactions in natural populations of Vibrio crassostreae. She completed her PhD at ETH Zurich, focusing on the deimmunization of endolysins targeting Staphylococcus aureus for therapeutic application and the structural and functional characterization of the phage φ26 38A endolysin.
Matthew Dunne was a senior scientist at ETH Zurich, where he led protein and phage engineering projects and collaborated with Lea on investigating φ2638A and its endolysins. Matthew is now CSO at Micreos Pharmaceuticals, where he leverages his background in biochemistry, protein biology, and R&D leadership to advance endolysins as precision antimicrobials in dermatology and oncology. Outside of his main role, Matthew is a scientific advisor to academic groups and is a keen golfer and runner with a passion for translating scientific innovation into next-generation diagnostics and therapeutics.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |

