When we think of viruses and worms, we often imagine them as different worlds: viruses as microscopic, rapidly spreading infectious agents, and worms as larger parasites that can linger in the body for years. Even though viruses and parasitic worms have different routes of transmission, they sometimes meet in the same human host, where they start to interact. The review “Helminth infections affect host immune responses to viral infections and vaccines” in FEMS Microbiology Reviews discusses how the interactions between these two parasites can have profound consequences on health, disease outcome, and vaccination efficiency, as explained by Juan García-Bernalt Diego in this #FEMSmicroBlog. #FascinatingMicrobes
When parasitic worms meet viruses
Parasitic worms, also known as helminths, infect more than a billion people worldwide, particularly in low- and middle-income countries. Infections are usually chronic, lasting for years, during which the worms manipulate the host’s immune system to ensure their own survival.
They dampen inflammation, promote tolerance, and reshape immune responses, allowing them to live quietly within their hosts. On its own, this immune modulation may seem harmless. And in fact, sometimes it is even associated with reduced allergies or autoimmune diseases. But when helminths cohabit with other pathogens, such as viruses, their impact becomes more severe.
The review “Helminth infections affect host immune responses to viral infections and vaccines” in FEMS Microbiology Reviews examines what happens during co-infections of helminths and viruses, and how these parasites affect the success of anti–viral vaccines.
Why parasite infections matter for vaccines against viruses
Helminths create an immune environment that can dramatically alter the way a host fights viruses. For example, the immune system normally mounts strong antiviral responses driven by Th1 cells and cytotoxic T cells to destroy infected cells. Helminths, however, push the immune system into the opposite direction toward responses that suppress inflammation.
The modulated immune system can weaken the host’s ability to clear viruses and its immunopathology, which is the collateral damage caused by the immune system when trying to fight viral infections. This further reduces infection morbidity, as seen for a wide range of viruses, including hepatitis, HIV, and respiratory viruses. The outcome depends on the helminth and virus involved, the tissues they infect, and the stage of both infections.
Perhaps even more concerning is the impact of worm infections on the efficacy of vaccines against other pathogens. Vaccination relies on a strong and well-directed immune response. But if helminths reshape immunity, vaccines may not work that well.
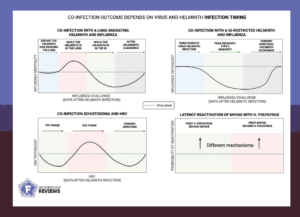
Indeed, people living with worm infections have reduced antibody levels and weaker T cell responses to vaccines against viruses like hepatitis B or influenza virus. This raises important questions for global vaccination campaigns. In the case of new vaccines, but also well-established ones, understanding how co-existing infections affect efficacy is crucial.
In some settings, public health-related interventions like mass deworming before vaccination might improve outcomes. However, their benefits on vaccination outcomes require further study.
Towards more effective virus control and vaccination worldwide
This review emphasizes that infectious diseases should be understood within the broader context of ecological and immunological interactions, requiring an integrative research approach. From a public health perspective, investments in parasite control may have spillover benefits on viral disease management.
As we aim not only to control but to eliminate certain diseases, it is essential to consider the role of helminths. Worm infections may undermine progress if they are ignored. But if taken into account, they could accelerate viral control campaigns.
Future studies should aim to identify which viral infections and vaccines are most influenced by helminth infections, elucidate the underlying molecular mechanisms, and evaluate whether interventions such as deworming or adjuvant-enhanced vaccination strategies can restore vaccine efficacy.
Above all else, understanding the interplay between worms and viruses is about protecting individuals in endemic regions and strengthening a global health system that is both resilient and equitable. Only this ensures that vaccines and treatments are effective for all populations, regardless of geography or coexisting infections.
- Read the review “Helminth infections affect host immune responses to viral infections and vaccines” by García-Bernalt Diego et al. in FEMS Microbiology Reviews (2025).

Juan García-Bernalt Diego is currently a postdoctoral researcher in the Virology Group at the Instituto de Investigación Hospital Universitario 12 de Octubre (Madrid, Spain), supported by a Sara Borrell fellowship. Juan’s research focuses on characterizing host-pathogen interactions and developing and characterizing next-generation vaccine platforms against emerging viruses, including SARS-CoV-2 and Ebola. His interest in immunology and vaccinology began during his Biotechnology studies at the University of Salamanca, where he also completed his PhD on diagnostic tools for parasitic infections. Since then, he has shifted from translational parasitology to fundamental and applied research in viral immunity and vaccine design.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |



