#FEMSmicroBlog: About the interactions between sugars from mother’s milk and the baby’s microbes
Milk sugars from the mother play a crucial role in the development of a baby’s gut microbiome. To explore the interactions between different sugars and the gut microbes in an infant, the study “Milk and mucin glycans orchestrate a synthetic infant gut microbiota structure” in FEMS Microbiology Ecology applies an in vitro synthetic community consisting of glycan degraders and cross-feeders. Maryse Berkhout explains in this #FEMSmicroBlog. #FascinatingMicrobes
Human milk sugars and mucin glycans: strikingly similar
Human milk oligosaccharides are among the most important components of breast milk. These sugars are not digested by the infant, but instead reach the microbes in its gut. Here, the milk sugars are food for the developing infant gut microbiome, and specifically stimulate the growth of beneficial bacteria, such as Bifidobacterium species.
However, human milk oligosaccharides are not the only sugar source that infant gut microbes feed on. Gut cells secrete a slimy mucus layer composed of mucins, which have a protein core with complex glycan extensions. Some gut microbes, such as Akkermansia muciniphila, can specifically feed on these glycans, with a large portion of their genomes dedicated to degrading mucin glycans.
Interestingly, human milk oligosaccharides and mucin glycans are similar in terms of building blocks and structure. As a result, gut bacteria use similar enzymes to degrade them and may even have overlapping glycan-degrading capabilities. Yet, it remains unclear how the availability of different infant gut glycans affects the development of the baby’s gut microbiota.
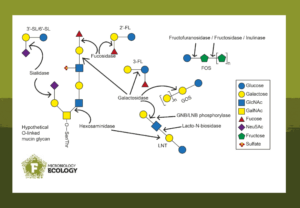
BabyBac: a developing infant gut synthetic community
The study “Milk and mucin glycans orchestrate a synthetic infant gut microbiota structure” in FEMS Microbiology Ecology investigated microbial interactions that may occur in vivo. For this, researchers hand-picked glycan-degrading bacteria to form the synthetic community “BabyBac” and explored its dynamics with different glycans.
BabyBac consists of
- Mucin glycan degrader Akkermansia muciniphila
- Human milk oligosaccharide degraders Bifidobacterium infantis and Phocaeicola vulgatus
- Human milk oligosaccharide and mucin glycan degraders Bifidobacterium bifidum and Ruminococcus gnavus
- Cross-feeders Escherichia coli and Blautia producta
Feeding different combinations of glycans from the infant gut to the synthetic BabyBac community affected the abundance of the microbes and their metabolite production. For example, even though Akkermansia muciniphila can degrade human milk oligosaccharides in single culture, it was only able to survive in the synthetic community when mucin was present.
Conversely, when mucin was the predominant glycan in the culture, Akkermansia muciniphila dominated the community. This reaffirms that this bacterium is highly specialised towards utilising mucin glycans.
When feeding BabyBac human milk oligosaccharides, glycan-degraders Bifidobacterium bifidum, Phocaeicola vulgatus and Ruminococcus gnavus thrived. However, Escherichia coli and Blautia producta only survived through cross-feeding. Because they cannot utilise the human milk oligosaccharides themselves, they require the sugar degradation products from others.
Infant gut microbes like it sweet
In addition to mucin glycans and human milk oligosaccharides, the effects of galactooligosaccharides and fructooligosaccharides on BabyBac were also tested. These sugars are often added to infant formula milk to stimulate the growth of beneficial gut bacteria.
Indeed, galactooligosaccharides and fructooligosaccharides had similar effects on the synthetic BabyBac community as human milk oligosaccharides. In this case as well, Phocaeicola vulgatus was the predominant glycan degrader with cross-feeding microbes also thriving. This shows that galactooligosaccharides and fructooligosaccharides have similar prebiotic effects on a microbial community as human milk oligosaccharides, highlighting the importance of adding prebiotic fibres to infant formula.
This study showed that complex sugars play crucial roles in the development of a healthy baby gut microbiota. It further highlights the importance of studying microbial functions in the context of a community.
Even though some bacteria contain glycan-degrading enzymes and can grow on a substrate in monoculture, they may not be able to compete with specialists within a community. Using synthetic communities to explore microbial ecology is a robust method, as it is highly replicable, versatile, and straightforward.
- Read the article “Milk and mucin glycans orchestrate a synthetic infant gut microbiota structure” by Berkhout et al. in FEMS Microbiology Ecology (2025).

Maryse Berkhout is a researcher in the Laboratory of Microbiology at Wageningen University & Research in The Netherlands. As part of the Microbiome, Mucus and Milk team, she studies microbial glycan degradation and ecology by applying synthetic communities.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |

