Bacteria often face rapidly changing and hostile environments. To cope, they rely on intricate stress-response networks. One of their survival strategies is nucleoid condensation—bacteria compacting their chromosomes to conserve resources. The study “The small bacterial membrane protein YohP induces nucleoid condensation in E. coli and inhibits oligomerization of antimicrobial peptides” published in microLife describes how the small membrane protein YohP helps the bacterium overcome stress, as explained in this #FEMSmicroBlog by Ana Natriashvili. #FascinatingMicrobes
The unknown protectors of the bacterial membrane
Small membrane proteins represent a substantial subset of proteins found in genomes of nearly all organisms. However, despite their abundance, the functions of only a handful have been characterized.
In bacteria, the levels of small membrane proteins often change with growth conditions; for example, during the transition into stationary phase, when nutrients become limited. During this period, small membrane proteins, like YohP, are strongly upregulated.
A previous publication showed that YohP changes the physiological state and lipid composition of the Escherichia coli membrane. At the same time, the protein triggers the stringent response—a protective adaptation that slows growth and reprograms metabolism, allowing Escherichia coli to conserve energy and improve stress resistance.
The study “The small bacterial membrane protein YohP induces nucleoid condensation in E. coli and inhibits oligomerization of antimicrobial peptides” published in microLife explored the molecular mechanisms of how this protein protects bacterial DNA under stress conditions.
Stress in the membrane shapes bacterial nucleoids
When entering the stationary phase, Escherichia coli starts producing YohP. As two copies insert as a dimer into the membrane, the membrane potential dissipates, activating the cellular stress response. This results in decreased global protein synthesis and cell viability.
Importantly, membrane depolarization is sufficient to trigger nucleoid condensation, which was specific to YohP and another small membrane protein, YncL, and was absent in cells expressing a control membrane protein. Moreover, YohP-induced nucleoid condensation occurred independently of nucleoid-associated proteins that usually regulate chromosome organization according to growth phase and stress conditions.
Membrane depolarization caused by YohP resembles the effects of magainin-2. This antimicrobial peptide, produced by vertebrates, inserts into the bacterial membrane as an oligomer. Here, it forms pores, disrupts the membrane potential, and eventually causes cell death.
The study showed that YohP-producing Escherichia coli cells were hypersensitive to magainin-2. The small membrane protein and antimicrobial peptide likely have an additive effect on the Escherichia coli membrane.
However, in YohP-producing cells, magainin-2 failed to induce nucleoid condensation, possibly due to the drastic loss of membrane potentiall. As the membrane is internally affected by YohP and externally by magainin-2, ATP- dependent enzymes, important for chromosome architecture, decrease.
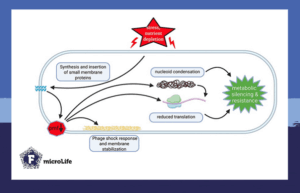
Moreover, in the presence of YohP, magainin-2 did not form pores in the membrane, probably because the membrane potential is needed for this. However, small pores can likely still form as magainin-2 interacts with lipid molecules in the membrane. Therefore, reduced oligomerization of magainin-2 in the presence of YohP does not necessarily impair the ability of magainin-2 to dissipate membrane potential.
Reduced fitness as a survival strategy
Although often overlooked, stress-induced small membrane proteins can reshape bacterial physiology by altering membrane properties, energy metabolism, and chromosome organization.
This study links nucleoid condensation by YohP and YncL directly to their abilities to depolarize the membrane. By compacting their nucleoids, bacteria reduce gene expression, protect their DNA, save energy, and thus, rapidly adapt to adverse conditions. Consequently, the reduced fitness of Escherichia coli becomes a strategic trade-off that enhances survival under harsh conditions.
- Read the article “The small bacterial membrane protein YohP induces nucleoid condensation in E. coli and inhibits oligomerization of antimicrobial peptides” by Natriashvili et al. in microLife (2025).

Ana Natriashvili completed her PhD at the Institute of Biochemistry and Molecular Biology at the University of Freiburg, where her research focused on small membrane proteins and their roles in bacterial stress responses and membrane organization. She earned her first master’s degree in Molecular Immunology and Microbiology at Tbilisi State University in Georgia while working at the Eliava Institute of Bacteriophage Microbiology and Virology. There, she developed a strong interest in alternatives to antibiotics, working on the isolation and characterization of bacteriophages from environmental samples and contributing to the development of phage cocktails targeting mono- and polymicrobial infections.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |

