Inducible promoters are everywhere in bacterial biotechnology but optimizing them remains a challenge. This blog, written by Andrés Felipe Carrillo Rincón, explores how he and co-author Natalie G. Farny approached this in their paper “Unlocking the strength of inducible promoters in Gram-negative bacteria“
Rethinking Old Paradigms
For decades, bacterial molecular biology relied on two central concepts:
First, the -10 and -35 promoter boxes recruit the sigma factor to initiate transcription. However, this knowledge largely remained confined to textbooks as a theoretical background, with little practical application. Second, plasmids are considered static extrachromosomal elements, with their copy number fixed and determined solely by the origin of replication.
These two concepts set the stage for developing a new generation of bacterial expression systems. By challenging these assumptions, researchers have unlocked the potential of virtually any promoter and developed plasmids with tunable copy numbers. Together, these innovations promise to transform laboratory productivity by enabling the use of multiple bacterial chassis, optimizing recombinant protein expression, and rendering molecular biology workflows more efficient.
The Power of the -10 and -35 Boxes
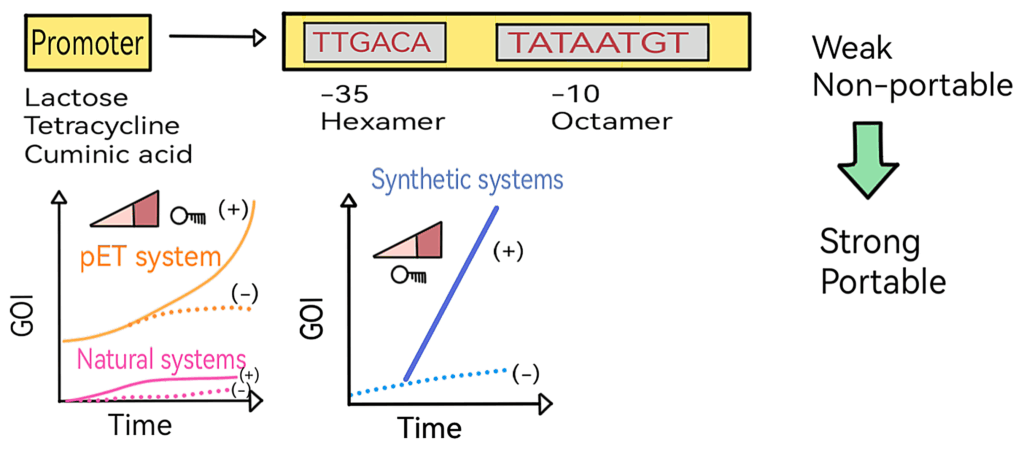
Initially described as hexamers, the -10 (Pribnow box) and -35 elements act as recognition sites for the sigma factor to initiate transcription. Through evolution, the primary bacterial sigma factor, σ70, became especially attuned to two consensus sequences: the -10 octamer (TATAATGT) and the -35 hexamer (TTGACA).
Strikingly, however, no natural promoter carries this perfect pair. Such a design would lead to excessive σ70 recruitment, resulting in uncontrolled overexpression of the downstream gene and draining cellular resources.
Instead, evolution produced imperfect -10 and -35 boxes that limit σ70 affinity. Transcriptional regulators then emerged to further modulate promoter accessibility and prevent unnecessary activation. The lac operon exemplifies this strategy, balancing efficiency with control.
The lactose promoter illustrates this evolutionary compromise. Its -10 and -35 elements deviate substantially from the σ70 consensus, ensuring that the right amount of lactose-metabolizing enzymes is produced only when needed. While this design is elegant in nature, it restricts the lac promoter’s utility in biotechnology, where large-scale protein expression is often required.
Addressing these limitations of natural expression systems naturally led to the idea of redesigning the σ70 recognition boxes.
Engineering Synthetic Promoters
To produce sufficient levels of the recombinant protein, synthetic biology approaches relied on the introduction of the perfect -10 octamer and -35 hexamer into natural promoters. By engineering these elements into systems such as the lactose and tetracycline promoters, their transcriptional strength rose to levels comparable with the pET system—a landmark in molecular biology.
Synthetic σ70 promoters offer one critical advantage: unlike the pET system, which requires specific E. coli strains, these synthetic promoters are portable. They function effectively in alternative hosts such as Vibrio natriegens and Pseudomonas putida while maintaining high productivity.
However, greater promoter strength came at a cost: transcriptional leakage. Even in the absence of induction, genes were expressed at detectable levels. The solution came through riboswitches, which allow precise regulation and ensure that promoters remain tightly “off” until deliberately activated.
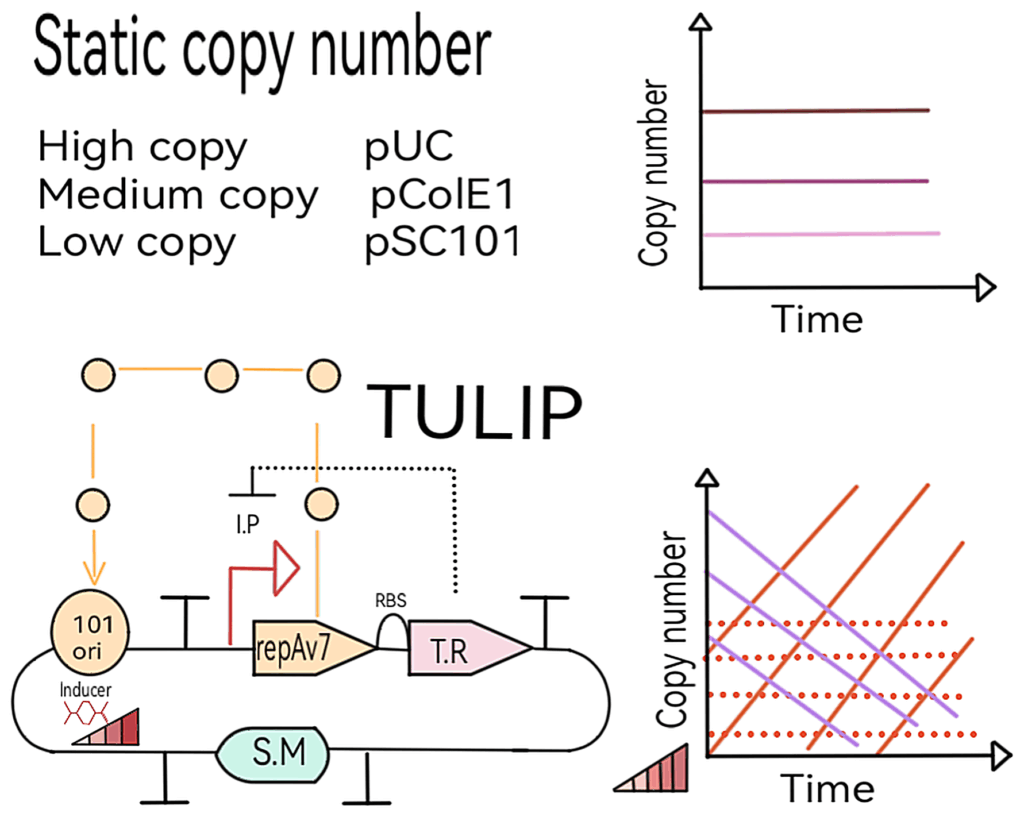
From Fixed to Flexible: Dynamic Copy Number Control with TULIP
Traditionally, expression systems relied on plasmid backbones with fixed copy numbers. For instance, pSC101 plasmids yield low expression since one cell contains only 1–5 copies, while ColE1 plasmids provide higher levels with one cell containing 20–30 copies.
When expressing toxic proteins, difficult-to-fold products, or metabolic enzymes intended for pathway engineering, fine-tuning is crucial. Producing just enough protein avoids overwhelming the cell while optimizing yield.
Historically, to achieve this balance, researchers needed to test countless combinations of promoters, plasmid backbones, and bacterial hosts. High-throughput approaches have become popular for this purpose, but they are resource-intensive and dependent on costly automation.
The TUnable Ligand Inducible Plasmid (TULIP) offers an elegant alternative. Derived from pSC101, TULIP enables researchers to control the number of copies in a bacterial cell, ranging from 1 to 200. This flexibility is achieved through a mechanism that regulates plasmid replication machinery directly by controlling the expression of RepA. With TULIP, precise expression levels can be achieved without resorting to exhaustive combinatorial testing.
The Ultimate Gene Expression Toolkit
Combining synthetic promoters with tunable plasmids provides researchers with a universal toolkit for gene expression. In a single plasmid system, researchers can now replicate the behavior of virtually all known plasmid backbones while retaining fine-grained control over expression.
- Need high yields of recombinant protein? Deploy TULIP at 200 copies per cell with a synthetic σ70-affinity promoter.
- Working with a toxic protein? Use TULIP at a single copy, combined with a synthetic σ70 promoter and a riboswitch for strict repression.
- Engineering a metabolic pathway? Combine multiple σ70 promoters and modulate expression of each enzyme individually.
These next generation bacterial systems are powerful, tunable, portable, and user-friendly. They remove the need for specialized host strains, adapt seamlessly to different bacterial species, and simplify the process of optimizing expression. I believe that every molecular biology laboratory should keep these tools stocked in their –20 °C freezer, ready to drive the next wave of discovery.
About the author
 Andres Felipe Carrillo-Rincon is a research scientist in the NetBio group at New York University Abu Dhabi. His work focuses on developing genetic tools that enable bacteria—the ultimate natural chemists—to produce next-generation recombinant proteins and valuable natural compounds. Felipe is a DNA and microbial engineer passionate about harnessing the full potential of nature through the power of synthetic and molecular biology.
Andres Felipe Carrillo-Rincon is a research scientist in the NetBio group at New York University Abu Dhabi. His work focuses on developing genetic tools that enable bacteria—the ultimate natural chemists—to produce next-generation recombinant proteins and valuable natural compounds. Felipe is a DNA and microbial engineer passionate about harnessing the full potential of nature through the power of synthetic and molecular biology.

