Pathogenic bacteria have evolved many clever ways to survive and thrive inside the human body. One such pathogen is Yersinia enterocolitica, a close relative of the plague bacterium Yersinia pestis and a cause of foodborne illness. To infect its host, Yersinia relies on the type III secretion system, a molecular syringe that injects specialized proteins, called effectors, directly into host cells. The article “In situ analysis of type III secretion chaperone proteins indicates a cytosolic handover of virulence effectors” in FEMS Microbes sheds light on one molecular aspect of this protein secretion system: chaperones. On this #FEMSmicroBlog, Katherine Pintor explains why bacteria need these specialized helper proteins for infection. #FascinatingMicrobes
The bacterial type III secretion system
Effectors secreted by the type III secretion system (T3SS) carry diverse enzymatic functions. Once inside the host, they can hijack cell processes, weaken immune defenses, or reprogram the cell for better bacterial proliferation.
Two well-studied effectors in Yersinia are YopE and YopH. While YopE disrupts the host’s cytoskeleton, YopH interferes with immune signaling.
Similar to other bacterial protein section systems, some T3SS effectors require small helper proteins called chaperones. These protect effector proteins from degradation and keep them ready for secretion. In Yersinia, two known chaperones fulfill this role: SycE for YopE and SycH for YopH.
Although chaperones usually interact with effectors, it remained unclear whether they also escort them to the injectisome, the T3SS apparatus, during secretion. To address this, the study “In situ analysis of type III secretion chaperone proteins indicates a cytosolic handover of virulence effectors” in FEMS Microbes investigated the roles of SycE and SycH in live Yersinia. Proximity labeling and single-particle tracking techniques were used to analyze the proteins’ interactions, localization, and mobility.
T3SS effectors need their cognate chaperones for delivery
According to the results, SycE is indispensable for the stability and secretion of YopE. Without SycE present, Yersinia enterocolitica was not able to neither secrete nor translocate YopE into host cells. This finding shows that chaperones are not peripheral helpers but critical determinants for effector stability and successful T3SS delivery.
Although SycH and SycE belong to the same class of T3SS chaperones, their behaviors diverge. SycH stabilizes YopH while it also interacts with negative regulators of the T3SS, linking it to T3SS regulation.
In comparison, SycE binds tightly to YopE. However, SycE showed weaker interactions with other exported proteins. This may reflect binding promiscuity, or effector clustering under secretion-inducing conditions.
To better understand their behavior during protein export, the study relied on mobility studies, single-particle tracking, and proximity labeling. Neither chaperone formed stable associations with the sorting platform components or injectisome.
Yet, both chaperones diffused more slowly when bound to their cognate effectors. Especially during secretion, mobility of the complex slowed significantly, but was not completely abolished.
Rewriting the T3SS model story
As the chaperone SycH does not interact with the sorting platform in vivo, the chaperone-effector complex is likely incorporated into larger protein complexes. Together, these results show that this T3SS chaperone functions entirely in the cytosol, stabilizing its designated effector until handing it over to the transport machinery.
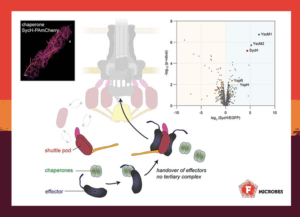
These findings support a new model of T3SS effector delivery: chaperones act as bodyguards in the cytosol, protecting effectors until their release. Rather than docking at the injectisome, they hand off the effectors to mobile shuttle complexes, which complete delivery to the T3SS. This handover step, once thought to occur at the injectisome, seems to happen even earlier in the cytosol.
It would be exciting to test whether other T3SS chaperones follow the same mechanism or use alternative pathways. This would ultimately deepen our understanding of how pathogens fine-tune effector delivery to manipulate host cells during infection.
- Read the article “In situ analysis of type III secretion chaperone proteins indicates a cytosolic handover of virulence effectors” by Pintor et al. in FEMS Microbes (2025).

Katherine Pintor conducted her doctoral studies in the group of Prof. Dr. Andreas Diepold, which investigates the molecular mechanisms and dynamics of the Type III secretion system in Yersinia enterocolitica. Her research focused on the chaperones SycE and SycH, using protein-protein interaction approaches to study their dynamics and cellular localization. She is now a postdoctoral fellow at the University of California, Berkeley, where her work centers on how Listeria monocytogenes secretes specific microbial ligands from the riboflavin pathway that stimulate the activation and expansion of MAIT cells.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |

