For millennia, we have employed fungi’s ability to make ethanol in culinary activities such as baking and brewing. Today, yeast-based ethanol production as biofuel is one of the largest biotechnological industries, with over 100 billion liters of ethanol produced globally per year. The study “Optimizing the balance between heterologous acetate- and CO2-reduction pathways in anaerobic cultures of Saccharomyces cerevisiae strains engineered for low-glycerol production” in FEMS Yeast Research combined two engineering strategies in a single yeast strain to boost ethanol production. In this #FEMSmicroblog, Aafke van Aalst discusses a sustainable way of ethanol production. #FascinatingMicrobes
Common strategies to produce ethanol in yeast
Since humankind is highly dependent on ethanol, making its microbial production more efficient could save millions of dollars and help biofuels compete with fossil fuels. To increase the yield of a product, one usually focuses on reducing the byproducts of the metabolic pathway.
In the ethanol production process, fungi, such as yeast, generate CO₂ and ATP. As ATP helps form biomass, this produces an excess of NADH. To maintain redox balance, yeast produces glycerol to convert NADH back to NAD⁺.
Researchers have therefore focused on improving ethanol yield by reducing either microbial biomass or glycerol formation. Reducing biomass yield has the potential to cause economic trade-offs as surplus yeast biomass is sold in animal feed products. Therefore, strategies to reduce glycerol formation are more interesting.
Metabolic pathways for ethanol production
Through glycolysis, glucose is converted into ethanol in a redox-neutral way. To maintain redox balance, Saccharomyces cerevisiae consumes the NADH formed during glycolysis when reducing acetaldehyde to ethanol.
However, by bypassing the NADH-producing step of glycolysis through a non-oxidative route, ethanol can be formed while consuming NADH. The enzymes phosphoribulokinase (PRK) and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) form the basis of the non-oxidative pentose phosphate cycle that incorporates CO2. In this pathway, CO2 serves as an electron acceptor, thereby coupling carbon flux to ethanol production with net NADH consumption.
Additionally, the bacterial enzyme acetaldehyde dehydrogenase (A-ALD) converts acetyl-CoA into acetaldehyde, while recycling NADH. Hence, by introducing this enzyme and providing acetate, yeast converts acetate into ethanol using the NADH-dependent acetate reduction pathway.
Understanding these NADH-dependent pathways highlights how substrate composition influences ethanol production efficiency. In industrial settings, such as the US ethanol industry, corn mash is the main feedstock, providing high glucose concentrations of up to 300 g/L but only a small amount of acetate of about 1.2 g/L. This composition is important because it limits how each metabolic pathway can contribute to ethanol production.
The PRK–RuBisCO pathway enables ethanol production with net NADH consumption and incorporates CO2, contributing to a more sustainable carbon flux. However, it operates only on glucose, not acetate.
In contrast, the acetate-reduction pathway converts acetate into ethanol. Yet, in corn mash, the small acetate content is insufficient to replace all the glycerol yeast normally produces, which is about 12–15 g/L.
Redox engineering to improve ethanol production
Since these two metabolic pathways complement each other, the study “Optimizing the balance between heterologous acetate- and CO2-reduction pathways in anaerobic cultures of Saccharomyces cerevisiae strains engineered for low-glycerol production” in FEMS Yeast Research aimed to engineer a yeast strain that could use both.
The acetate-reduction pathway would metabolize the present acetate to boost ethanol yields. When acetate runs out, the PRK-RuBisCO pathway would fix CO2 and re-oxidize NADH. In principle, this dual-pathway approach could maximize substrate utilization and minimize glycerol formation throughout the full fermentation process, regardless of acetate availability.
However, combining two NADH-consuming systems in one cell introduced a new challenge: competition for the same redox cofactor. If one pathway outcompetes the other, the potential benefits might not be fully realized.
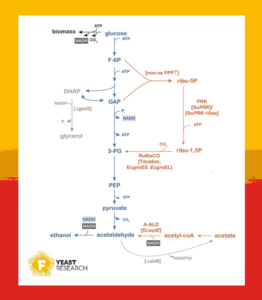
Fine-tuning the activity of each reduction pathway seems to be key: strong enough to be effective, but not so strong as to interfere with the other. The engineered yeast in this study reduced glycerol formation by 55%, while ethanol yields were 6% higher compared to non-engineered yeast.
This work demonstrates that, for first-generation feedstocks, combining CO2– and acetate-reduction strategies can optimize ethanol production. Importantly, careful balancing of these competing pathways is necessary to prevent metabolic bottlenecks. This opens the door to more efficient, sustainable biofuel production in fungi.
- Read the article “Optimizing the balance between heterologous acetate- and CO2-reduction pathways in anaerobic cultures of Saccharomyces cerevisiae strains engineered for low-glycerol production“ by van Aalst et al. in FEMS Yeast Research (2025).

Aafke van Aalst is an industrial microbiologist fascinated by how microbes can be engineered for sustainable production. During her PhD at TU Delft, the Netherlands, she worked with controlled bioreactors and engineered yeast for improved bioethanol production. This experience sparked her interest in using dynamic pathway regulation to optimize microbial processes. As a postdoc at DTU, Denmark, she studies and applies these strategies to further improve bioproduction. Aafke enjoys designing experiments that push the limits of microbial productivity and translating fundamental research into real-world applications.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |
