Antibiotic-resistant bacteria are a growing threat to global public health. Alone in 2019, antimicrobial resistance was associated with nearly 5 million deaths worldwide. Under certain conditions, even sensitive bacteria can survive antibiotic exposure resulting in relapsing infections when therapy is discontinued. Understanding the mechanisms that bacteria use to survive or even proliferate in the presence of antibiotics is obviously critical to developing more effective therapeutic strategies. The recent short review “Role of (p)ppGpp in antibiotic resistance, tolerance, persistence and survival in Firmicutes” in microLife highlights the critical function of a specific bacterial stress response in antibiotic resistance as presented by Andrea Salzer for the #FEMSmicroBlog. #FascinatingMicrobes
The bacterial stringent response
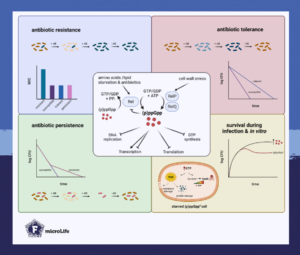
To survive and multiply under unfavorable environmental conditions, microbes have evolved strategies to sense and respond to various stresses. The stringent response is one such adaptive mechanism as it enables bacteria to survive under nutrient starvation, antibiotic exposure and other related stresses.
When a bacterium encounters such a challenging condition, its intracellular (p)ppGpp levels rise and immediately shut down most metabolic processes and growth. As the effector molecule of the stringent response, (p)ppGpp also reprograms bacterial physiology through transcriptional changes that ultimately reallocate cellular resources and ensure the survival of the bacterium.
Antibiotic resistance, tolerance and the stringent response
Firmicutes, a group of Gram-positive bacteria that includes several important human pathogens, make exceptional use of the stringent response. For example, methicillin-resistant Staphylococcus aureus (MRSA) is responsible for difficult-to-treat infections in humans and caused more than 100,000 deaths attributable to antimicrobial resistance in 2019.
This pathogen acquired a genetic determinant that encodes penicillin-binding proteins with low affinity to methicillin and other β-lactam antibiotics. Hence, even in the presence of β-lactam antibiotics, cell wall biosynthesis can continue and the bacterium is resistant. By increasing the production of penicillin-binding proteins, the stringent response even determines the level of resistance.
The stringent response also contributes to antibiotic tolerance; a condition at which sensitive bacteria can survive antibiotic exposure without being resistant. In fact, most antibiotics target active metabolic processes. By inducing an immediate shut-down of metabolism and growth, high (p)ppGpp levels render the bacteria insensitive to the actions of antibiotics.
One notable example here is the biocide triclosan, which is widely present in consumer products such as toothpaste or antimicrobial hand wash. As a response to low concentrations of this biocide, the stringent response allowed S. aureus to become increasingly tolerant to triclosan.
The stringent response in antibiotic persister cells
Despite a clear link between the stringent response and antibiotic tolerance and resistance, it is still controversial what role (p)ppGpp plays in the formation of persister cells. These represent an antibiotic-tolerant subpopulation of cells within a generally antibiotic-susceptible population.
In some Firmicutes, elevated (p)ppGpp levels are linked to an increased antibiotic tolerance within a subpopulation. Yet, in other species, the involvement of the stringent response in antibiotic persistence could be clearly excluded.
Distinct from antibiotic persistence, the term “persistent infection” refers to difficult-to-treat infections that are not cleared by the host immune system. Persistent pathogens are difficult to eradicate as they deploy many evasion strategies to the standard antimicrobial therapy. For example, they use the stringent response or learn to cope with unfavorable environmental conditions within the host.
In several nosocomial pathogens, mutations leading to elevated (p)ppGpp levels have been associated with persistent infections. These mutations might have been selected because they provide the pathogen with the ability to evade the host immune response or to tolerate antibiotics.
Overall, the review “Role of (p)ppGpp in antibiotic resistance, tolerance, persistence and survival in Firmicutes” in microLife discusses how bacteria use the stringent response to evade antibiotic attacks. The article highlights the importance of continued research in this area and outlines the potential of (p)ppGpp as a target for new antibiotics.
- Read the short review “Role of (p)ppGpp in antibiotic resistance, tolerance, persistence and survival in Firmicutes” by Salzer and Wolz et al. (2023).

Andrea Salzer studied biochemistry at the University of Tübingen. Currently, she is carrying out a PhD at the Interfaculty Institute of Medical Microbiology and Infection Medicine (University of Tübingen) in the group of Prof. Christiane Wolz. Andrea is studying the stringent response and its role in bacterial survival in the human pathogen Staphylococcus aureus.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |
