The human gut is a natural reservoir for the opportunistic fungal pathogen Candida albicans. In a healthy gut environment, the indigenous microbiota may prevent pathogenic overgrowth and the spread of infection via a process called colonization resistance. The article “Human gut bifidobacteria inhibit the growth of the opportunistic fungal pathogen Candida albicans” in FEMS Microbiology Ecology describes bacterial species common in the adult gut microbiota and how their activities hamper the growth of C. albicans in vitro. Liviana Ricci explains for the #FEMSmicroBlog how these findings have potential for new therapeutic means for Candida infections. #FascinatingMicrobes
The problem with Candida
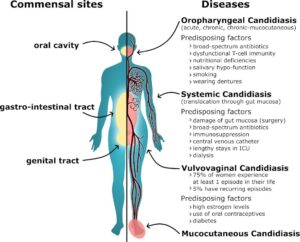
Candida albicans inhabits the guts of many people, ordinarily without affecting their health. However, in immunocompromised patients dissemination from the gut can lead to serious disease.
Candida infections are a significant healthcare burden. With rising numbers of elderly/frail individuals as well as increasing resistance to existing anti-fungal drugs, this challenge may even worsen in the coming years.
To counter this burden, alternative therapeutic strategies are needed to reduce Candida load in the gut and its potential systemic dissemination. An attractive target to start with is the gut microbiota.
The gut microbiota as a potential source of novel anti-Candida therapeutics
The human gut microbiota prevents pathogens like C. albicans from inhabiting or overgrowing in the human gut. For that, resident microbes use the so-called colonisation resistance mechanism to both stimulate the host’s immune defense and directly compete with the fungus.
Therefore, the study “Human gut bifidobacteria inhibit the growth of the opportunistic fungal pathogen Candida albicans” in FEMS Microbiology Ecology aimed to identify specific gut microbiota species with anti-C. albicans properties and their underlying mechanisms.
Bifidobacterium species inhibit the growth of C. albicans in vitro
First, C. albicans was co-cultured with faecal microbiota samples from six healthy individuals under anaerobic conditions mimicking the human colonic environment. The study found that some co-cultures strongly suppressed the growth of C. albicans.
Next, 16S rRNA gene sequencing profiled the bacterial communities in the co-culture incubations. Bifidobacteria, and Bifidobacterium adolescentis in particular, were most correlated with increased inhibition of C. albicans. Subsequent testing with a variety of representative gut anaerobes confirmed the strong inhibitory properties of bifidobacteria, particularly that of B. adolescentis, against C. albicans.
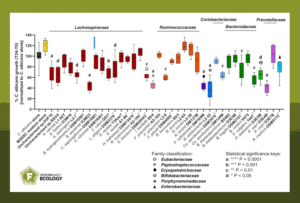
Secreted fermentation acids and low pH to inhibit Candida
The study further investigated the mechanisms that underpin C. albicans suppression. The combination of releasing fermentation acids into the bifidobacterial culture supernatants and the resulting slightly acidic pH seem to be key for the inhibition of C. albicans growth.
The fungus was largely unaffected by the pH drop alone and moderately resistant to single and mixed fermentation acids produced by bifidobacteria. However, growth was impaired when C. albicans was cultivated in the presence of both stressors.
The fermentation acid concentrations that inhibited C. albicans according to this study are physiologically relevant for gut regions such as the proximal colon. This suggests that total fermentation acids may also represent a key mechanism of colonization resistance against C. albicans in vivo.
It is noteworthy that B. adolescentis is present in about half of adult Westernized populations and its growth is stimulated by dietary resistant starch. This study, therefore, opens up possibilities for subsequent in vivo studies. For example, it would be interesting to determine if antagonistic species such as B. adolescentis and their activities could be viable targets for novel bio-therapeutic or dietary intervention strategies to reduce the risk of C. albicans overgrowth.
- Read the article “Human gut bifidobacteria inhibit the growth of the opportunistic fungal pathogen Candida albicans” by Ricci et al. (2022).
- Read also “#FEMSmicroBlog: A call to increase the representation of non-European cohorts in gut microbiome research”
Liviana Ricci is a postdoc in the Computational Metagenomics lab (University of Trento, Italy) where she studies human uncharacterized bacterial species and mechanisms of horizontal transmission of non-pathogenic bacteria. She completed her PhD in the Gut Microbiology group of the Rowett Institute (Aberdeen, UK) where she focused on in vitro characterization of single microbial species and of microbe-microbe and microbe-host interactions. She is interested in bacterial metabolic functions and their participation to host health and defense from infections.
About this blog section
The section #FascinatingMicrobes for the #FEMSmicroBlog explains the science behind a paper and highlights the significance and broader context of a recent finding. One of the main goals is to share the fascinating spectrum of microbes across all fields of microbiology.
| Do you want to be a guest contributor? |
| The #FEMSmicroBlog welcomes external bloggers, writers and SciComm enthusiasts. Get in touch if you want to share your idea for a blog entry with us! |
